Research >> Research Interest | Research Grants
Bottleneck of nanomedicine
Therapeutic nanoparticles are under intense development for the treatment of diseases (e.g., cancer, cardiovascular diseases) (Allen et al., Science, 2004). Through rational design of nanoparticle properties, they can enhance and sustain the delivery of drug payload to desired biological sites (Davis et al., Nat. Rev. Drug Discov., 2008). Current progress lies on perturbing the physicochemcial properties of nanomaterials (e.g., size, charge, shape, targeting ligand density) to achieve desired clinical outcomes (e.g., circulation, stability, toxicity, efficacy). For any therapeutic to exert its intended effect, and given the high cost and synthetic difficulty of pure and potent compounds, precise delivery to targeted sites is imperative. Yet, not much research effort has been directed to elucidate the mechanisms of delivery.
Despite proven success in delivery in a few organs (e.g., tumor, liver), reports on successful targeted delivery to other sites still remain scarce. From our previous work, intravenously (i.v.) injected gold nanoparticles of different sizes in the sub-micron size range (20 – 150 nm) do notnaturally accumulate inside organs except the liver and spleen (Fig.1; Choi et al., PNAS, 2011). The mere deposition of 1-2% of the injected dose in the kidney, heart, lung, and pancreas highlights the biological barriers that obstruct efficient nanoparticle entry, restricting the delivery of actual therapeutic nanoparticles for treatment of diseases arisen from these sites. This realization prompts our research group to question if nanostructures, when strategically engineered, can accumulate in these “challenging organs” in appreciable quantities.
Our approach: Dissection of basic "nano-bio" interactions
To design novel and functional nanostructures for targeted delivery to challenging biological sites in vivo, our research group believes the importance of dissecting the basic interactions of nanoparticles with biological structures across the vast length scales of organ, tissue, and cell. Take our previous work on kidney targeting as the first example.
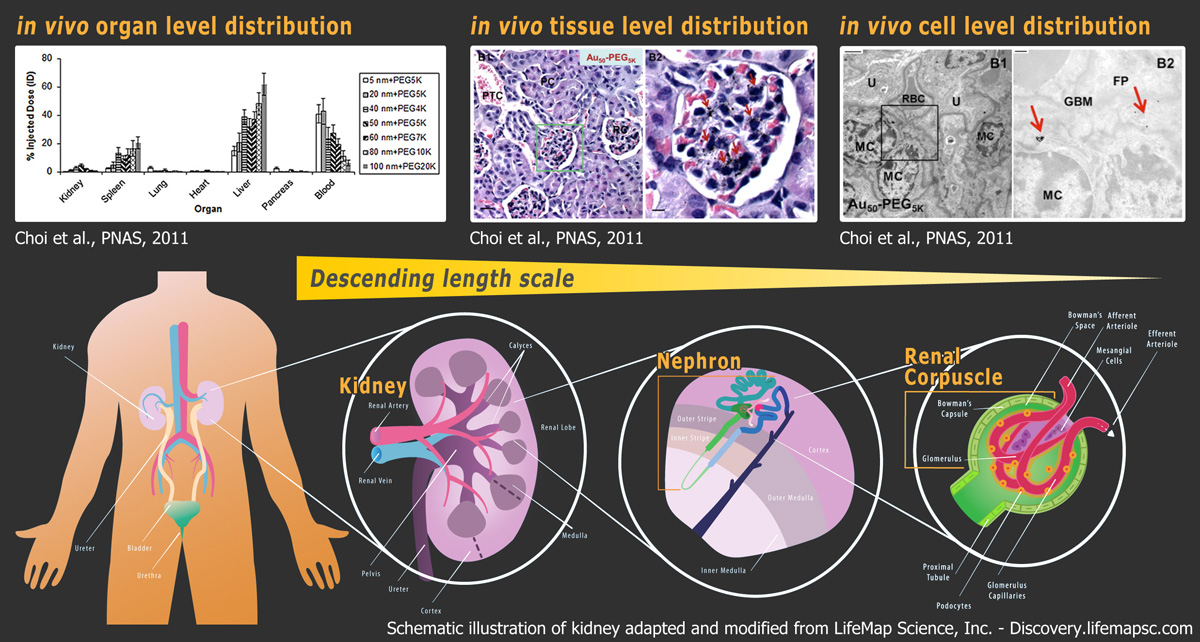
I. Organ-level distribution As an example, we will explore how gold nanoparticles coated with poly(ethylene glycol) (PEG) will interact with the kidney. After 24 hours of injection, most intravenously injected nanoparticles in the 20-150 nm size regime deposit in the liver and spleen. Intriguingly, there is up to 5% of the injected dose collecting in the kidney. Specifically, such renal deposition is size-dependent: there exists an optimal size range of nanoparticles (i.e., 75 ± 25 nm) in which deposition is maximized. Knowing how nanoparticles interact with the tissue structures within the kidney will aid our understanding in this size-dependent phenomenon.
II. Tissue-level distribution All injected PEGylated gold nanoparticles collect inside renal corpuscles (green box), which are filters of blood for production of urine. Significantly, 75 ± 25 nm nanoparticles (red arrows) can be located in every renal corpuscle, and none can be observed inside the surrounding renal tubules. To investigate the high efficiency for targeting renal corpuscles, knowing how these 75 ± 25 nm nanoparticles interact with the constituent cells of renal corpuscles will be crucial.
III. Cellular-level distribution Despite the absence of active targeting ligands, 75 ± 25 nm nanoparticles can still enter and accumulate inside mesangial cells inside renal corpuscles. Mesangial cells can secret anti-inflammatory cytokines and internalize foreign biomacromolecules. We speculate that these mesangial cells serve as the primary source of nanoparticle retention inside the kidney.
In summary, collective analysis of in vivo nanoparticle distribution across the length scales of organ, tissue, and cell can provide mechanistic insights into how nanoparticles interact with the biological system. These insights, in turn, will inform useful design rules for building nanoparticles that can specifically target defined biological destinations (in this case kidney mesangium.) These results may open up avenues for imaging or treating diseases that arise from that particular biological destination (in this case IgA nephropathy).